30+ Calculating Equivalence Point
The section of curve between the. Web x 01Kb1 2 746x106 OH pOH -log 746x106 513.

Why Do We Get Two Changes In Slope In Conductometric Titration Of Naoh Against Oxalic Acid Quora
Web Determine the initial pH the pH at the midpoint halfway to the equivalence point and the pH at the equivalence point if 2500mL of 01335 M NH3 NH4OH is.

. Web Recall that the equivalence point is defined to be the point at which the moles of titrant added is stoichiometrically equivalent to the amount of moles of the unknown. PK a 375. Web The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten when moles.
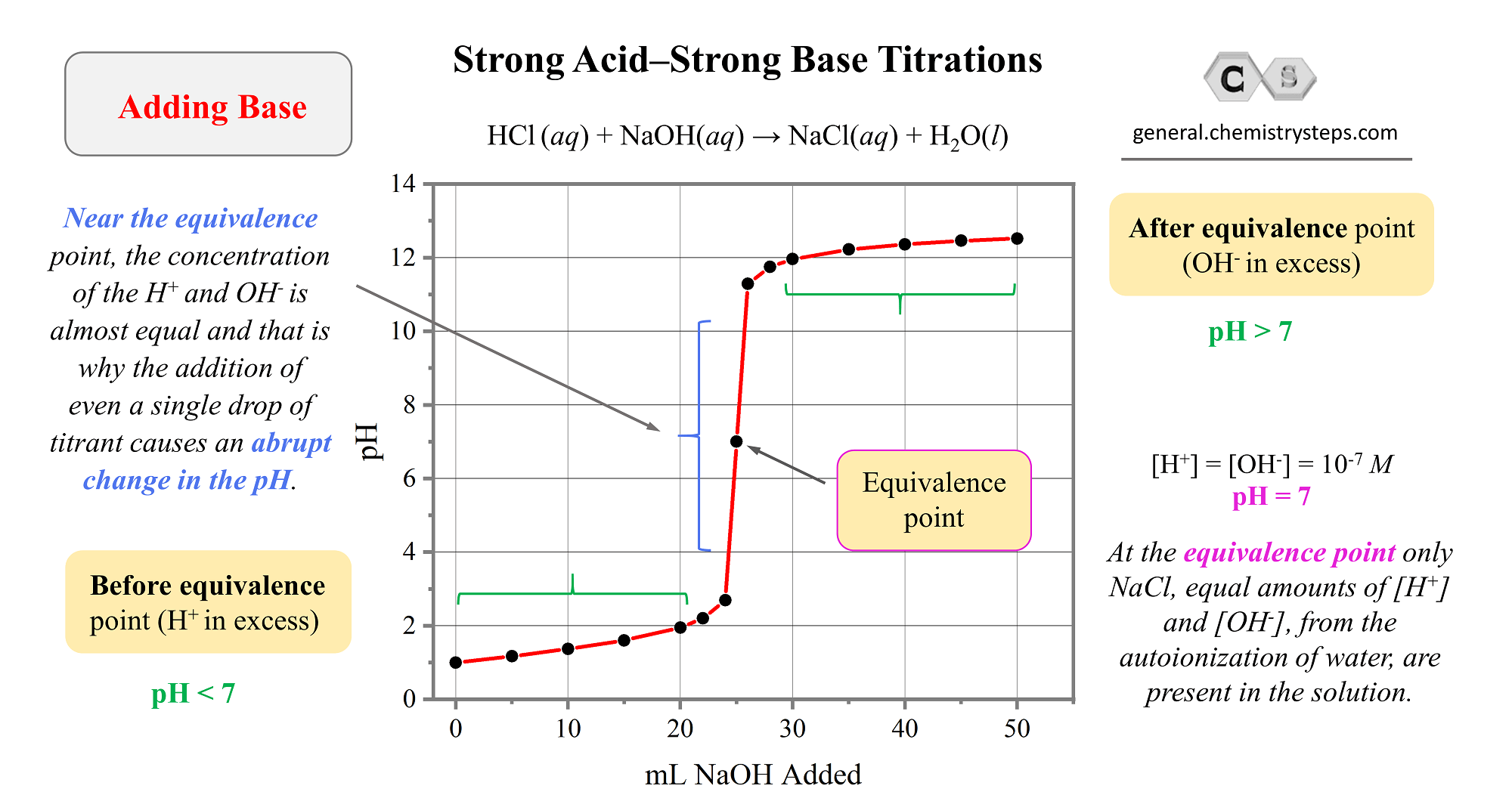
The moles of titrant. The solution pH is due to the acid ionization of HCl. Web In an acid-base titration the titrant is a strong base or a strong acid and the analyte is an acid or a base respectively.
In other words while titrating it is a point. Explain what an acid-base indicator is and how it works. For the titration of a weak acid with a strong base the pH curve is initially acidic and has a basic equivalence point pH 7.
Web Calculate pH at the equivalence point of formic acid titration with NaOH assuming both titrant and titrated acid concentrations are 01 M. Web The equivalence point of an acidbase titration is the point at which exactly enough acid or base has been added to react completely with the other. Determine the pH of resultant solution when 250 cm 3 of 01 moldm-3 NaOH.
Web Identify the equivalence point and half-equivalence points. Web In this JC2 webinar we want to learn how to calculate the pH at equivalence point. Web Solution a Titrant volume 0 mL.
PH 14 - pOH 887. When all of a weak acid has been neutralized by strong. Because this is a strong acid the ionization is complete and the hydronium ion molarity.
A point at which the moles of the titrant is equal to the moles of analyte in the solution Titrant. When solutions of some polyprotic acids are. The point in a titration when the titrant and analyte are.
Web The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Web The equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte solution. The substance or reagent with known concentration and volume.

Equivalence Point Labster

Calculate The Ph At The Equivalence Point Of A Weak Acid Strong Base Titration 005 Youtube
What Is Advertising Value Equivalency In Pr Ave Alternatives
Titration Of A Polyprotic Acids Chemistry Steps
Sin 30 Degrees Find Value Of Sin 30 Degrees Sin 30

17 3 Calculating The Ph At The Equivalence Point Of A Weak Acid Strong Base Titration Youtube

Titration Weak Acid Strong Base Before Equivalence Point Youtube
What Is The Shape Of Titration Curve When H2so4 In Burette Is Added To Naoh Is There Only 1 Equivalence Point What If H2so4 Is Changed To A Weak Diprotic Acid Like

Equivalence Point And Pi R Mcat
What Is Equivalent Annual Cost Eac Gocardless
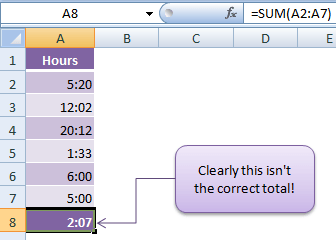
Calculating Time In Excel My Online Training Hub
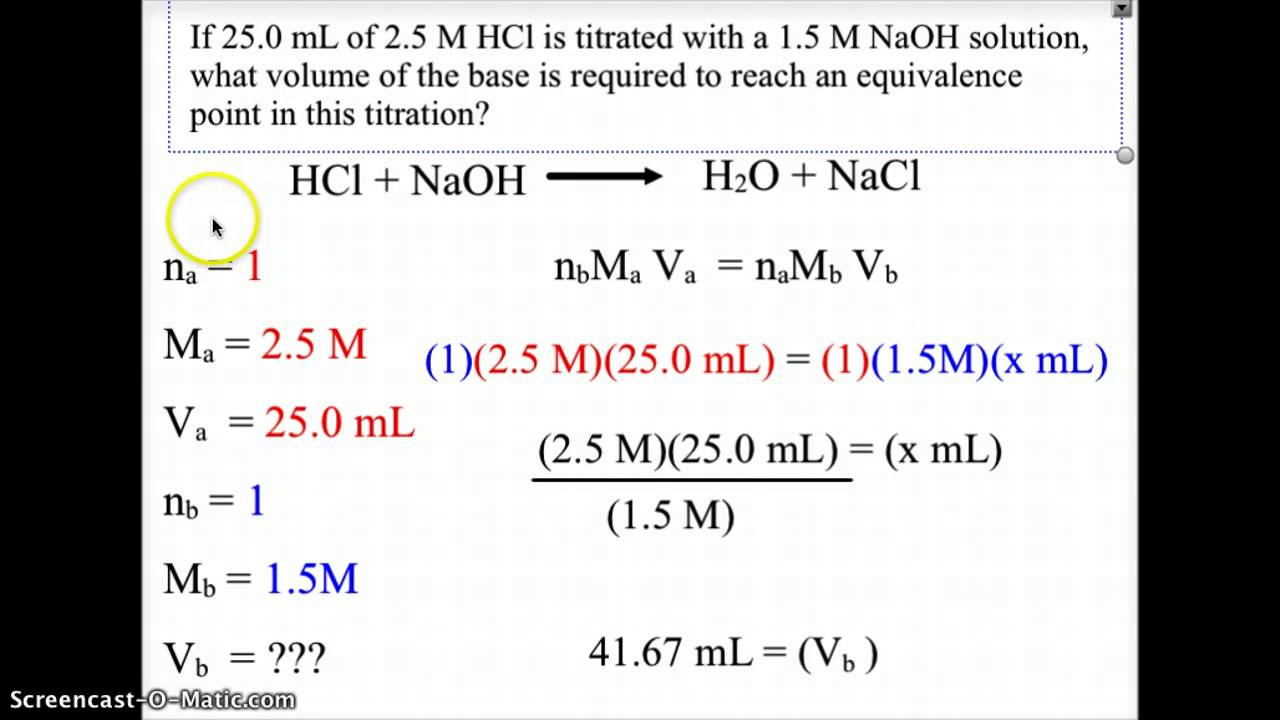
Acid Base Titration Equivalence Point Youtube

7 9 The Half Equivalence Point Youtube
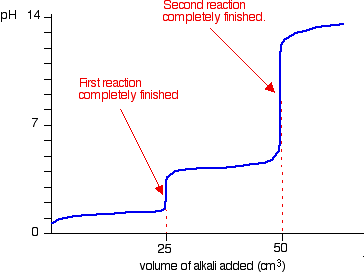
Ph Titration Curves Chemistry Libretexts

Titration Curves Equivalence Point Calculations Chemtalk

17 4 Titrations And Ph Curves Chemistry Libretexts
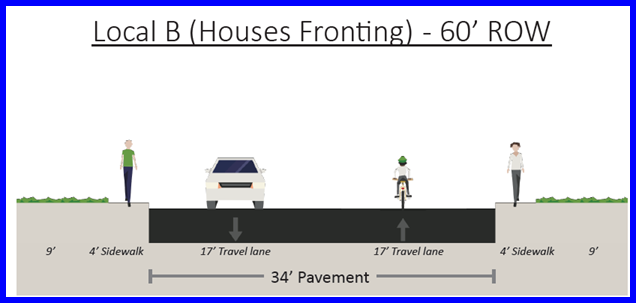
Article V Development Standards Unified Development Code San Antonio Tx Municode Library